Project Alexander
EU FP7 collaboration with Boehringer Ingelheim demonstrating the value of pre-screening in absorption models
Project Alexander: Boehringer Ingelheim
As part of the EU FP7 Project Alexander, we investigated "Compound X" for Boehringer Ingelheim. This was a compound that had been taken into animal testing because of promising in vitro data. However the compound did not perform as was expected, and no therapeutic effects were seen.
With our systems we were able to show that the compound was not absorbed in the model system, and that the nanoparticle delivery system did not improve absorption (Figure 1). This demonstrates the value of pre-screening in the absorptive model before testing potential compounds and formulations in animals – and the related cost implications.
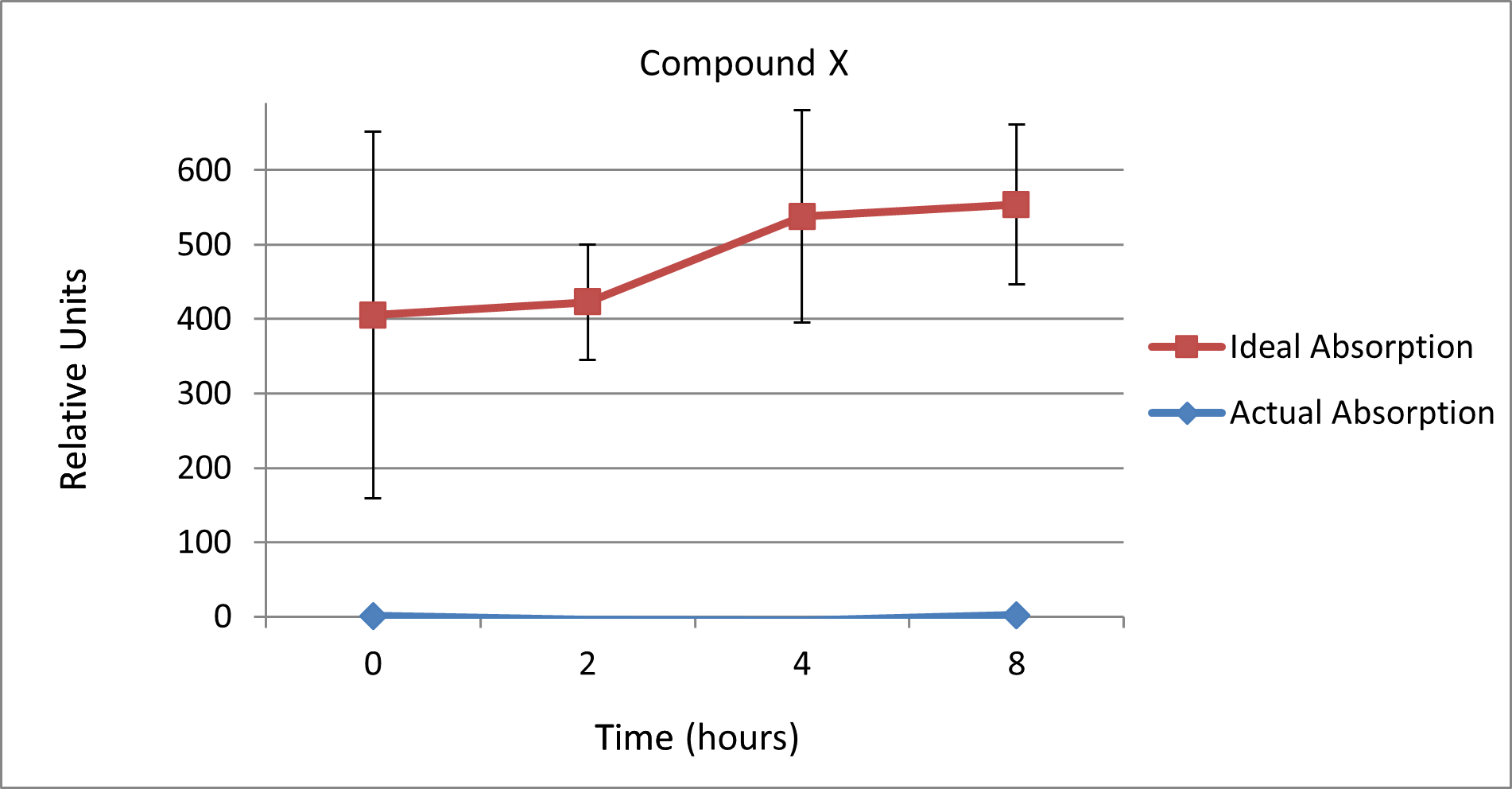
Figure 1 – Absorption of "Compound X" alone or in a nanoparticle delivery system through the epithelial model, as compared to an unimpeded absorption profile.
Screen Before You Test
Reduce development costs and animal testing by evaluating absorption in our predictive models first
Speak to an Expert

