Lipase Inhibitor Development
How our Digestion Model predicted clinical trial failure and saved millions in development costs
The Background
Lipases are a class of digestive enzymes responsible for digesting dietary lipids into fatty acids and glycerols for absorption. Inhibition of lipase can help treat obesity by preventing dietary fats from being absorbed and therefore reducing overall caloric intake and supporting weight management efforts.
For the development of a novel lipase inhibitor, it is essential to assess the activity of any candidate compound in a physiologically relevant environment as the compound must remain active in the complex environment of the GI tract. Therefore, Aelius Biotech's Digestion Model was used to assist in the development of a new class of lipase inhibitors.
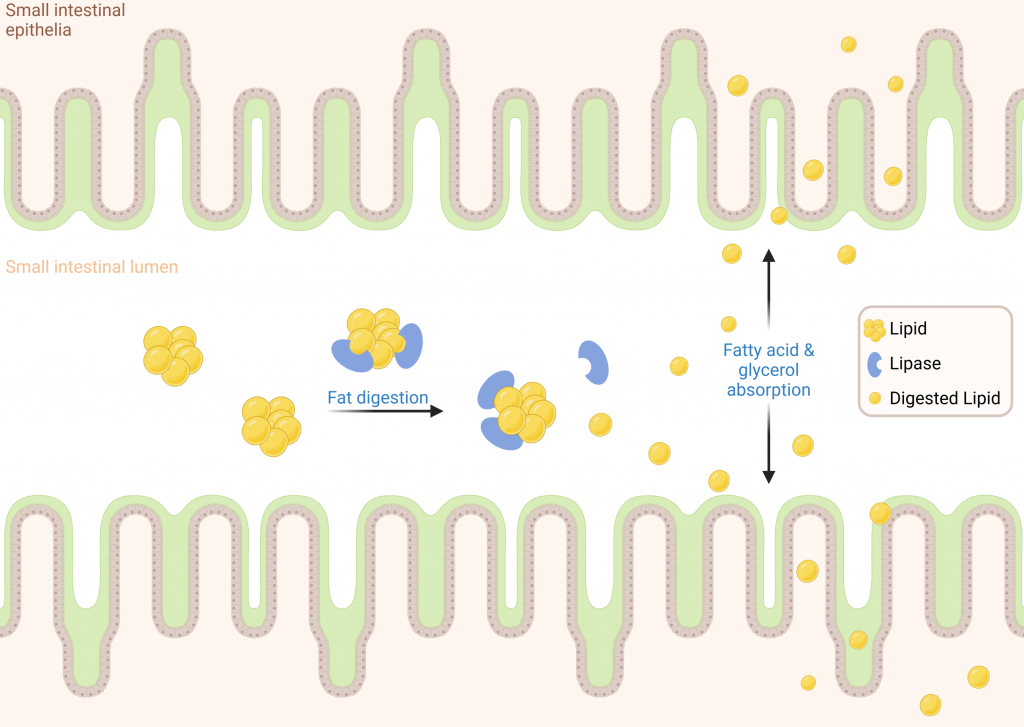
The Model
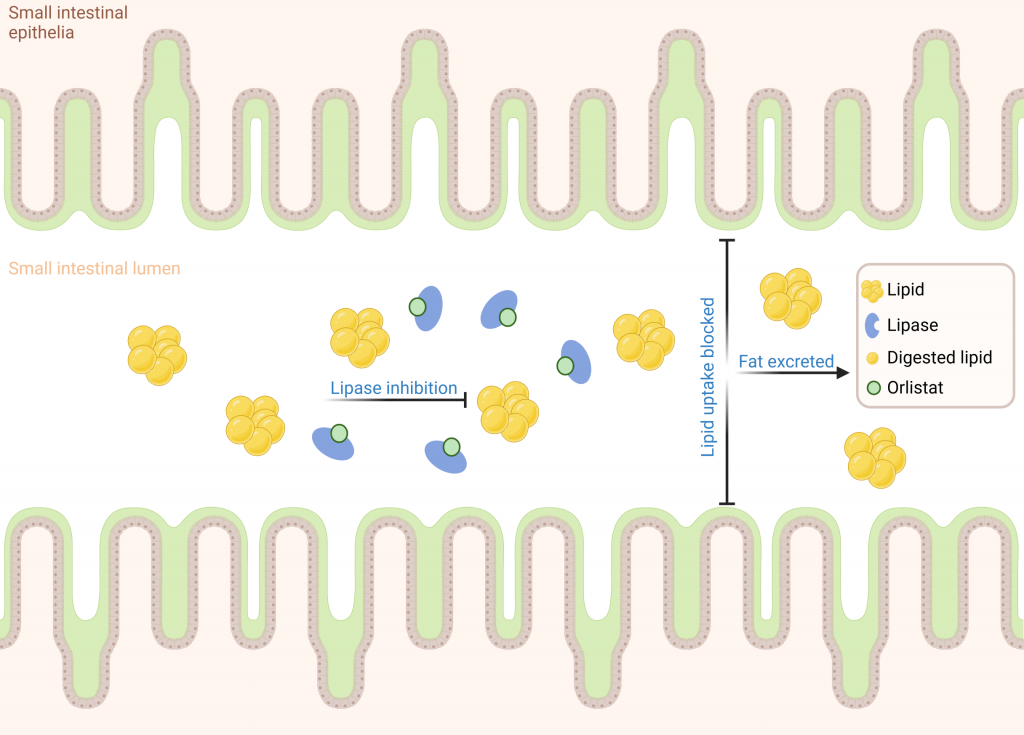
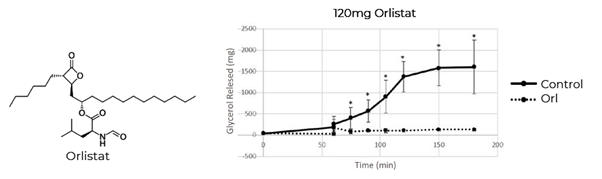
Orlistat, developed by Roche in 1983, is an OTC human pancreatic lipase inhibitor. Orlistat was used as a positive control in our models. In order to investigate the efficacy of inhibitors in the presence of food, a pork and chicken pie was digested in the Aelius Digestion Model with and without orlistat. Glycerol release was measured across the 3h time course as an indicator of fat digestion. As expected, the data showed substantial inhibition of glycerol release when orlistat was present when compared to the orlistat control demonstrating effective lipase inhibition (Figure 3).
The Problem
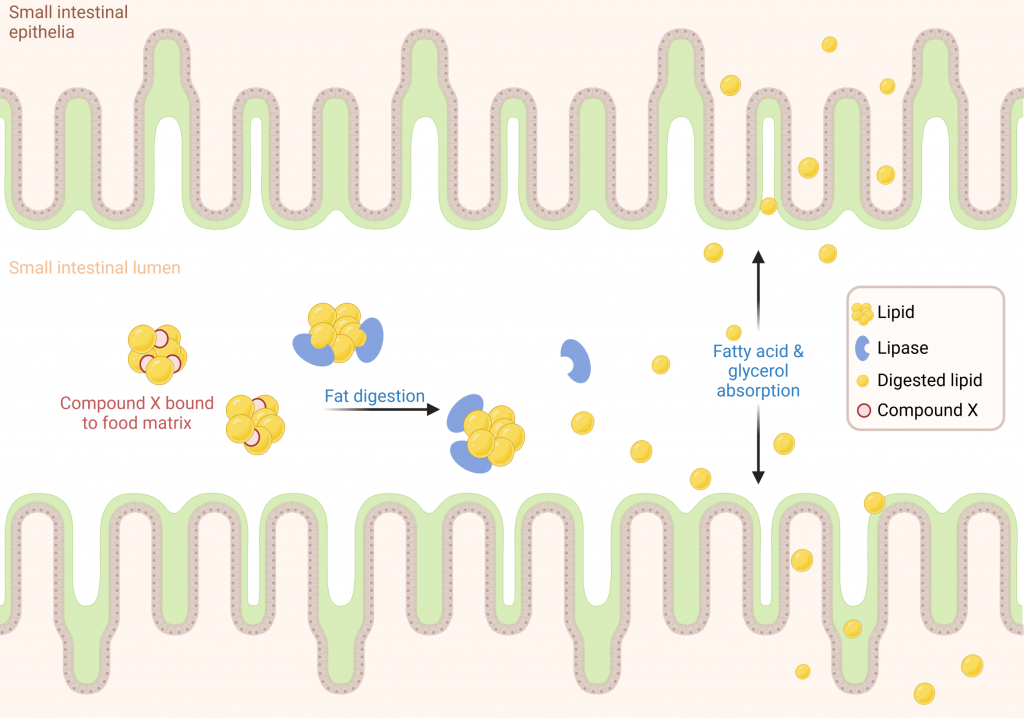
Client X had developed a novel lipase inhibitor with strong inhibition in isolated biochemical assays and had committed to a clinical trial. However, they had concerns about non-specific binding in the complex environment of the human digestive tract that could impact the activity in vivo (Figure 4).
The Solution
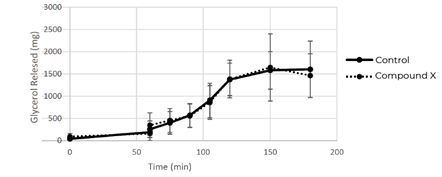
The lipase activity was assessed in the same manner as previously described and Compound X showed no inhibition of glycerol release when compared to the vehicle-only control (Figure 5).
Conclusion
The Aelius Digestion Model was used to demonstrate that lipase inhibitor compound X was inactivated in the presence of a meal. Unfortunately, Client X had already invested in a costly clinical trial, where their candidate ultimately showed no efficacy- as predicted by the Aelius Digestion Model. This outcome highlights the importance of evaluating a compound's efficacy in biorelevant environments, early in the development process. Had Client X utilised our models earlier, we could have identified this issue upfront, enabling them to redesign their compound to address the problem before moving forward with clinical trials.
Avoid Costly Clinical Failures
Test your compounds in physiologically relevant conditions before investing in clinical trials
Speak to an Expert

